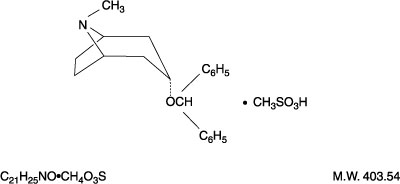
Benztropine
View Brand InformationWhat is Benztropine?
Top Global Experts
Related Clinical Trials
Summary: In this study, the investigators will examine whether a deprescription of unnecessary anticholinergic drugs (benztropine or trihexyphenidyl) can augment quality of life, functioning, and neurocognition in individuals who with schizophrenia. Individuals identified by clinical services who have unneeded prescriptions benztropine or trihexyphenidyl will be eligible for deprescription and study entry....
Summary: The goal of this clinical trial is to test if patient education or duloxetine can be used to treat multisystem functional somatic disorder (FSD). The main questions it aims to answer are: * Does duloxetine work better than placebo in the treatment of FSD? * Does patient education work better than usual treatment for FSD? * Does the combination of patient education and duloxetine work better than u...
Related Latest Advances
Brand Information